The laws of thermodynamics are some of the most fundamental laws of physics. They describe the way matter and energy move through the universe, so what is thermodynamics? Thermodynamics is the study of heat (thermo) and motion (dynamics), and its relationship to energy. These laws were not discovered in a single stroke of genius by one individual. Instead the accumulated knowledge of heat, motion and energy that led to the discovery and formulation of these fundamental laws happened gradually over time and was the result of the work of many individuals. Put another way, the laws of thermodynamics emerged gradually over time as the field of science progressed throughout the 18th century. Today there are four laws of thermodynamics – the first, second third, and zeroth law.
Discovering the Nature of Heat
People have learned how to make things hot since they first tamed fire, but never understood the true nature of heat. The concept of heat is a fundamental aspect in understanding how the universe works. A serious investigation of the nature of heat began during the industrial revolution. The massive economic gains as a result of the revolution provided the impetus for the discovery of these laws, with steam power being the hot topic of the day. People wanted to understand how steam could drive an engine and how to improve its efficiency.
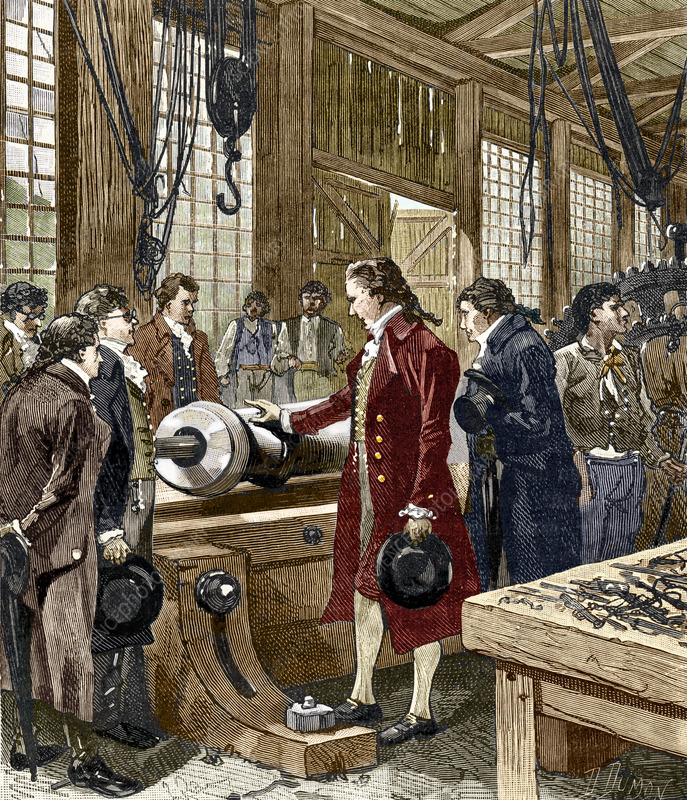
The solution to the problem came from Benjamin Thompson, also known as Count Rumford. While Count Rumford did not directly formulate the laws of thermodynamics, he provided the experimental data that was the basis for the laws. He did this through a series of experiments involving the boring of cannons. He observed that the temperature would rise as it was bored, and that this rise in temperature was proportional to the amount of work done in the boring process. He also observed that heat could be generated indefinitely, and this contradicted the competing caloric theory of heat.
Despite Rumsford’s experimentation’s the caloric theory survived for nearly another half century until the German Julius Mayer became the next person to relate mechanical work to heat quantitatively. Mayers work did not receive much attention shortly after the British scientist James Joule preformed additional experiments on the generation of heat by friction, electricity, and magnetism. The unit of energy, the Joule, is named in honor of his work. The eventual realization that heat is a form of energy was a critical step in the development of the laws of thermodynamics.
“Nothing in life is certain except death, taxes and the second law of thermodynamics.” – Seth Lloyd
Establishing the Laws of Thermodynamics
In 1824 a French military engineer named Sadi Carnot had expressed some of the ideas that would become the second law. This contribution came from his publication “Reflections on the Motive Power of Fire” where he analyzed the operation of idealized heat engines and introduced the concept of the Carnot Cycle.
It took a few decades for Carnot’s insights into the nature of heat to be formalized as the laws of thermodynamics. In the 1850s, Rudolf Clausius and William Thomson fully formulated what became known as the first two laws. It was Clausius who introduced the concept on entropy. The third law was formulated in the first decade of the 20th century by Walther Nernst. The zeroth law came last and was formulated by Ralph Fowler in the 1920s.
The zeroth law was discovered after the first three but named the zeroth law as it provides a frame of reference for the first three laws. The laws of thermodynamics are as follows.
- Zeroth Law – If two thermodynamic systems each are in thermo equilibrium with a third one, they are in thermal equilibrium with each other
- First Law – This is also known as the law of conservation of energy. It states that in a closed system the total energy remains the same and can only be transferred from one form to another. Therefore energy cannot be created or destroyed.
- Second Law – The entropy of an isolated system not in equilibrium will always increase over time, approaching a maximum value at equilibrium. The second law dictates the arrow of time.
- Third Law – As temperature approaches absolute zero, the entropy of a system approaches a constant value.
The laws of thermodynamics was a pivotal moment in scientific history. It revolutionized our understanding of heat and energy, and also laid the groundwork for many technological advancements from the Industrial Revolution to the present day.
Continue reading more about the exciting history of science!