Language is one of the special characteristics that distinguishes humans from other animals. It allows us to communicate complex concepts and ideas to other people, undoubtedly providing us with a remarkable evolutionary advantage over other species. Writing is a set of markings used to represent a language. It augments the benefits of language by making it permanent, allowing the message to travel further and persist through time. It is why the invention of writing systems often distinguish history from prehistory.
The History of Writing

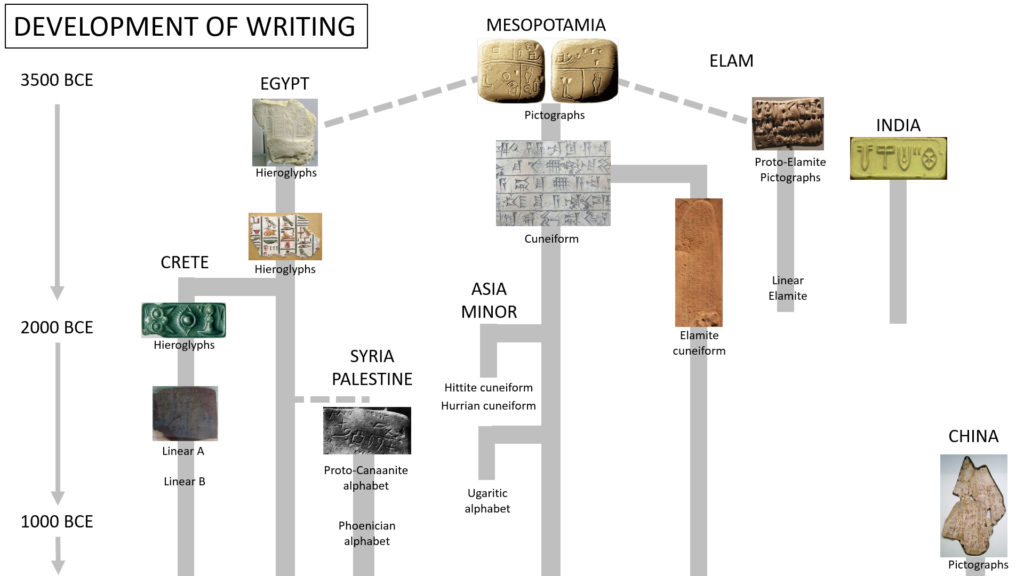
The history of writing systems traces a complicated journey and much of its detail is lost to time. Written language emerged around 3400 BCE in Sumer, southern Mesopotamia. These same industrious people also invented number systems and the wheel. Their writing form is known as cuneiform (cunea, Latin for “wedge”) and consisted of making wedges on clay tablets. It is derived from their proto-writing system of using clay tokens of various shapes as counters for various goods that were produced and exchanged, essentially an accounting system.
The original tokens date back to around 8500 BCE and the system evolved over the millennia in several stages of abstract symbolism. The earliest tokens were of the most basic geometric shapes such as a cone or a square, and later tokens took the shape of more abstract shapes such as miniature tools and fruit. Despite its complexity, each token was a unique geometric shape, such as a cone, and each one representing, with a one-to-one correspondence, a certain type of good. Two cones mean two baskets of grain. No matter your language, if you understood that a cone token meant a basket of grain you could account for the transaction. These tokens were most likely accounts of debt and were stored inside clay envelopes.
This brings us to the original purpose of the Sumerian writing system: accounting – in the recording amounts of grains, numbers of livestock, and various other goods. As the civilization grew in population size the number of debts increased. Since the tokens were stored inside envelopes their contents could not quickly be known until you opened the envelopes and counted the tokens. Some accountants solved this problem by making wedges on top of the envelopes representing the contents of the envelope. The transition from token to script begun and the worlds first writing system emerged. Eventually clay tablets with markings representing the tokens completely replaced the token system since the impression of the cone on the tablet was identical to the cone token itself.

(Credit: Wikimedia Commons)
It took around another 400 years until the Sumerian writing system made the shift to create the phonetic signs of speech. This was moving from a clear one to one representation to a more abstract for of representing sounds. This created a big problem for a society inventing a writing system. It has to agree upon a system of symbols or makings to represent spoken sounds. This agreement would take some time. Pictorial notations such as a picture of a bird or a tree were easiest to agree upon. Eventually consensuses were built and writing formats gradually became more formalized, arranging itself it to standardized rows and columns. The full development of the Sumerian writing system took at least 1,000 years.
(Credit: Wikimedia Commons)
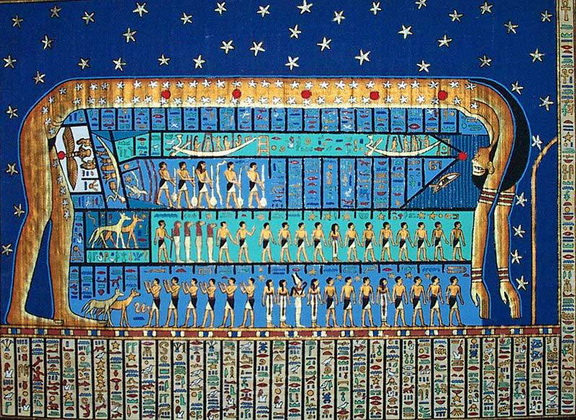
It is not certain whether writing originated in a single geographic area (Sumeria) and spread throughout the world by cultural diffusion or if it was invented in a few areas independently. The discovery of scripts in ancient Mesoamerica certainly seems to indicate that it was invented at least more than once. In the Old World it is very likely that only the Sumerians and a few centuries later the Egyptians independently invented their own writing system. It is also possible that the Egyptians borrowed the idea from the Sumerians, nobody knows for sure. The Egyptian writing system is called hieroglyphics (meaning “sacred engravings” in Greek) and are pictorial in form. There are about 1000 distinct characters. It is the most famous and well known ancient form of writing.
Good Ideas Like Writing Spread, Now This Good Idea Spreads Other Good Ideas
Due to the difficulty in inventing writing systems, it is likely that all writing systems have been borrowed and altered from early Mesopotamian writing systems with the exception of the Egyptian, Chinese and Mesoamerican writing systems. Writing systems also require a long time to fully develop, probably at least a thousand years. Other rudimentary writing systems may have been invented but they were either absorbed, aborted, or replaced due more the established writing systems rapid diffusion.
In the 16th century BCE the Canaanites simplified the Sumerian and Egyptian pictographic scripts by creating an alphabet of 22 consonants. All of our modern alphabets are derived from this script. Eventually the Greeks introduced characters for vowels, establishing the alphabet to be used for Western Civilization. Once writing spread across the globe itself became the means for spreading other good ideas. A fitting destiny for one of humanity’s most impactful ideas.
Continue reading more about the exciting history of science!